
The Science Behind Phosphor Light Emission
1. Introduction
Phosphorescence is a fascinating process of light emission by a substance following the absorption of energy. Unlike fluorescence, in which the emission of light is almost instantaneous, phosphorescence is characterized by a time-delayed emission of light. This delayed glow arises from the time it takes for the electrons in the material to move back to the ground state from an excited state. Phosphorescent materials have many practical applications in safety lighting, display devices, and even in art.
The history of phosphorescence has been traced to ancient times, with ancient descriptions of glow-in-the-dark substances being found in natural minerals. With advancements over time in the science of materials, there have been the discoveries of an extensive variety of inorganic and organic substances with phosphorescent ability. The materials currently make up a critical component of usage ranging from consumer electronics to emergency safety systems.
2. Working Principle of Phosphorescence
Phosphorescence begins with energy absorption, usually light. The energy gets the electrons in the material excited by transferring them from a low-energy location (ground state) to a high-energy location (excited state). Electrons transition to a higher orbit or energy level when taking in energy.
After the excitation, the electrons do not have the ability to move back to the ground state in a flash. They undergo a forbidden transition and thus require more time than regular fluorescent processes. The slow return gives rise to the release of energy in the form of light after a while, and phosphorescent materials contain an afterglow because of this.
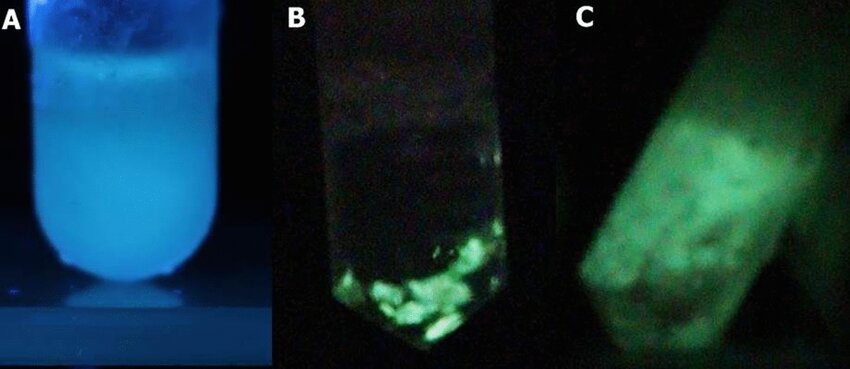
Phosphorescence and fluorescence of Deltatoria bremleii extracted material upon UV light irradiation: (A) fluorescence; (B) phosphorescence of pelleted material and debris after UV light irradiation; (C) phosphorescence of frozen supernatant. Viviani, Vadim. (2023). Biophosphorescence in fluorescent millipedes (Diplopoda: Xystodesmidae) and its relationships with bioluminescence. Scientific Reports. 13. 10.1038/s41598-023-47860-9.
3. Phosphorescence Emission Mechanism
Phosphorescence occurs when a substance absorbs energy and then emits light over a matter of time. This is in contrast to fluorescence, which takes place almost instantly. The unique feature of phosphorescence is that light is emitted after a delay due to the process of electron transition from one energy level to another, indeed by what is known as a "forbidden transition."
In phosphorescent materials, the electron is excited to a higher energy level first when the material is irradiated with light. Rather than returning to its ground state right away (as in fluorescence), the electron follows a more gradual transition as permitted by quantum mechanical constraints. The gradual return to the ground state is responsible for the long-lasting emission of light after the exciting source is removed.
Types of Phosphorescence Emission:
-
Single Phosphorescence Emission: This is the most common and straightforward form of phosphorescence. The material absorbs energy and then emits light in a single wavelength after some time lag. The duration of the emitted light is determined by the material's individual energy levels and transition times.
-
Fluorescence-Phosphorescence Dual Emission: Some materials exhibit both phosphorescence and fluorescence. In such materials, light is emitted initially very quickly (fluorescence) and later with a lag (phosphorescence). Double emission can be because the material sustains both singlet and triplet excited states. Both types of emissions from the material can be beneficial in applications such as displays, where there is a requirement of high speed response as well as long-lived afterglow effects.
-
Dual Phosphorescence Emission: This is an effect that occurs in certain advanced materials, where the material will emit two separate phosphorescent emissions at separate wavelengths. This could be due to the material having multiple triplet excited states that lead to different light emission results. Dual phosphorescence is useful for applications requiring greater than one color or better light control, such as multi-color displays or sophisticated lighting systems.
Phosphorescence emission also depends on external factors such as temperature and material purity. As the temperature is raised, the transition of the electrons is quicker, and the afterglow length will be shorter. The process will be interrupted, and the light emission will be altered by the impurities of the material, affecting the efficiency and the glow lifetime.
This appreciation of the various emission behaviors helps to account for the wide range of applications for phosphorescent materials, from a comparatively straightforward glow-in-the-dark use through to an advanced, multi-functional technology.
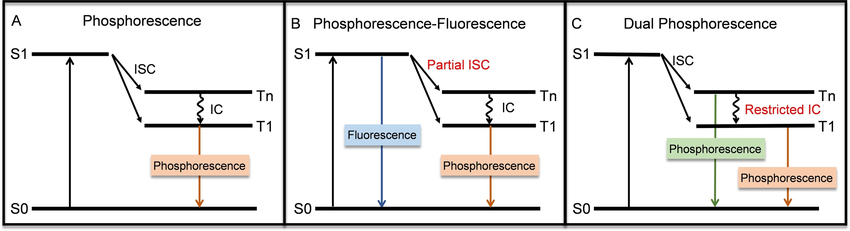
Emission mechanisms for (A) single phosphorescence emission, (B) fluorescence‐phosphorescence dual emission, and (C) dual phosphorescence emission. Ruan, Zhipeng & Yang, Jun & Li, Yonghua & Zhang, Kenneth. (2024). Dual‐Emissive Iridium(III) Complexes and Their Applications in Biological Sensing and Imaging. ChemBioChem. 25. e202400094. 10.1002/cbic.202400094.
4. Phosphorescent Decay Characteristics
Phosphorescent material possesses a special decay time, or the duration of time the material continues to emit light when the source of excitation is withdrawn. The afterglow is anywhere from several seconds to a few hours, depending upon the material as well as the environment.
Different materials have different decay times, and those decay times can be affected by outside conditions like temperature. At higher temperatures, electrons can return to the ground state in less time, shortening the decay time. The purity of the material also plays a role—impurities in the material can interrupt the process of energy transfer, thereby altering the time of light emission.
5. Types of Phosphorescent Materials
Phosphorescent substances come in various forms, each with unique properties and uses.
-
Inorganic Phosphorescent Materials: A few of the inorganic phosphorescent substances are alumino-silicates, yttrium aluminum garnet (YAG), among others. These substances are extensively used in blue and white light-emitting systems and are famous for their intensity and stability.
-
Organic Phosphorescent Materials: Organic molecules are now more commonly used for applications such as OLED displays and organic lighting. Organic molecules have the benefit of being more energy-efficient and flexible, so they are well suited for consumer electronics.
-
Rare-Earth Element Doped Materials: Doping rare-earth elements like europium or terbium in phosphorescent materials enhances the material's emission properties, where light color can be controlled more and there is higher efficiency in phosphorescence.
Ytterbium-doped Yttrium Aluminium Garnet (Yb:YAG)
6. Applications of Phosphorescent Materials
Phosphorescent materials find applications everywhere, ranging from numerous applications in today's technology:
-
Display Technologies: Phosphorescent materials play a key role in OLED (Organic light-emitting diode) displays, which are used in a variety of electronic devices, including televisions, smartphones, and wearables. These materials allow for vibrant colors and high energy efficiency.
-
Lighting: Phosphorescent materials are used in energy-efficient lighting solutions, including phosphorescent lamps and light panels. These materials are also used in emergency lighting systems to ensure visibility in low-light environments.
-
Safety Signage and Environmental Applications: Phosphorescent materials are crucial in safety systems, where they are used for signs, exit lights, and emergency indicators. These materials glow in the dark, providing vital guidance in power outages or other low-visibility situations.
-
Art and Decoration: Phosphorescent materials are also used in various artistic and decorative applications, adding a glowing effect to paintings, sculptures, and interior designs. Their ability to absorb light during the day and glow in the dark creates striking visual effects.
7. Conclusion
Phosphorescent materials are a fundamental part of modern technology, from everyday consumer electronics to specialized safety equipment. Their ability to absorb energy and re-emit light within a span of time makes them both practically and aesthetically desirable. As research on new materials and new modes of manufacture continues, the applications for phosphorescence will continue to multiply. In whatever form it appears in the next generation of display technology, energy-saving light, or in creating safe low-light environments, phosphorescent materials will be a crucial part of the technological landscape.
Stanford Optics provides high-quality phosphorescent materials, designed for vibrant and efficient light emission in advanced optical and display systems.





